When patients turn to healthcare providers, providers turn to Cassling.
We’re your trusted source for all of your imaging, surgical and therapeutic needs. We offer exceptional equipment service, digital health solutions and diagnostic imaging systems from Siemens Healthineers.
SPECIALTIES
Supporting Healthcare Providers in a Variety of Specialties
No matter your area of expertise, we’ll find the solution to fit your needs. As a provider, your practice requires customized clinical solutions to address the challenges of today and thrive in the ever-evolving environment of tomorrow. Find your specialty below to learn how Cassling can help you expand your patient base and succeed in a competitive landscape.
PRODUCTS & SOLUTIONS
The Innovations You Need to Succeed
You require a consultative approach to healthcare that ensures the right fit for both you and your patients. Here’s how we get you there.
Multiple Solutions. One Trusted Source.
Applications & Training
Consulting
Continuing Education
Equipment Service
Financing
Marketing
Project Management
Site Planning
TESTIMONIALS
Hear from our Clients
“For Cassling, the word I would choose to describe them is integrity. They’re not just about their own success; they want the customer to be successful too.”

Sharon Harms
"The service from Cassling has been excellent. We’ve built a partnership with Cassling over the years and we have an excellent relationship."

Jeff Bender
ABOUT CASSLING
For nearly 40 years, Cassling has built a reputation for excellence based on our commitment to customer service and improving community healthcare.
With an eye toward the future, we’ve harnessed our expertise and unique position to become a true partner in addressing the needs of modern healthcare, all while remaining an employee-centric, family-owned company. By 2030, we’ll have touched 350 million patient lives thanks to the dedication of healthcare providers like you.

175 Million Patient Lives and Counting
Featured Resources

Leadership Fundamentals: Preventing Burnout, Strengthening Morale and Boosting Productivity
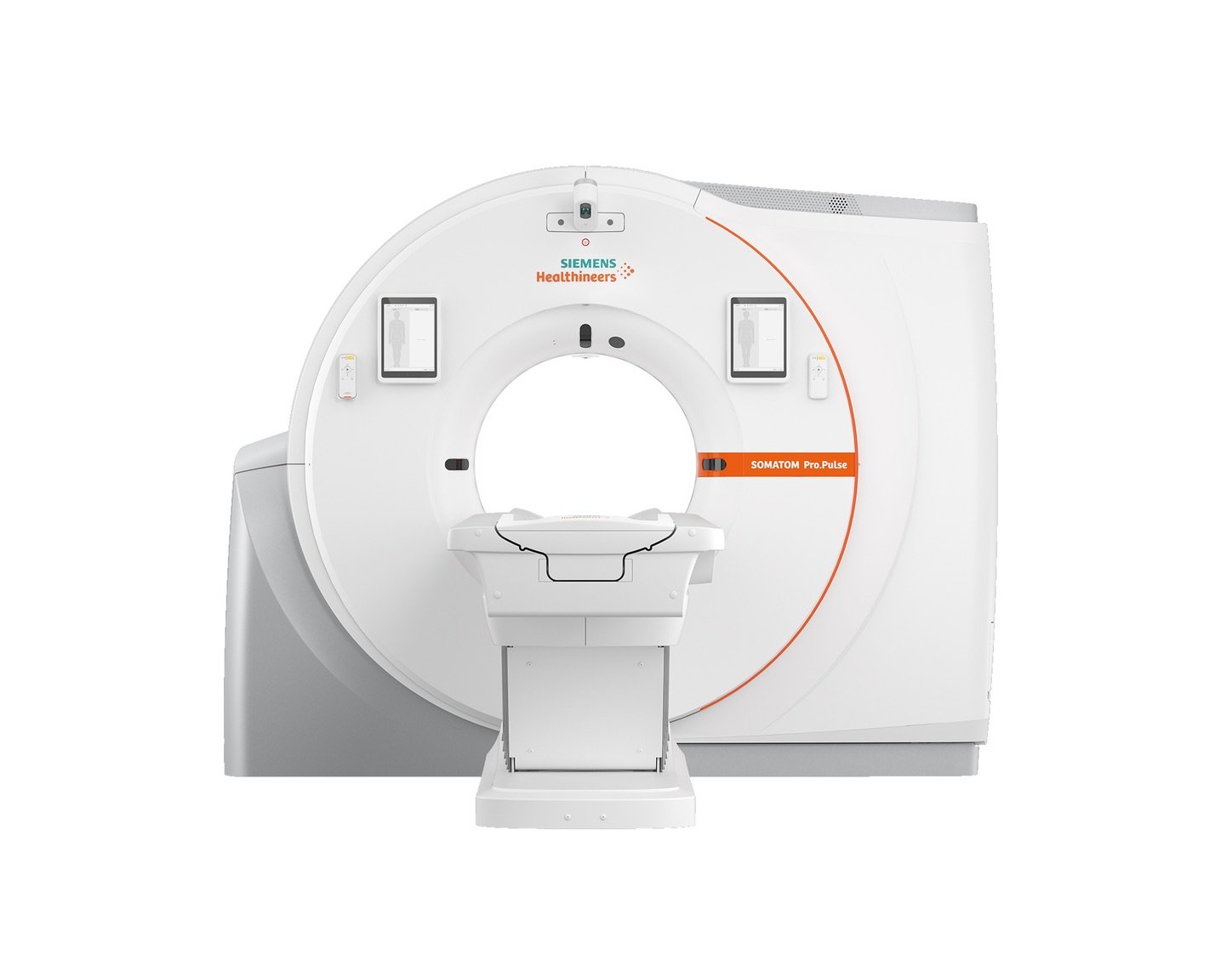
SOMATOM Pro.Pulse Dual Source CT Scanner Cleared by FDA
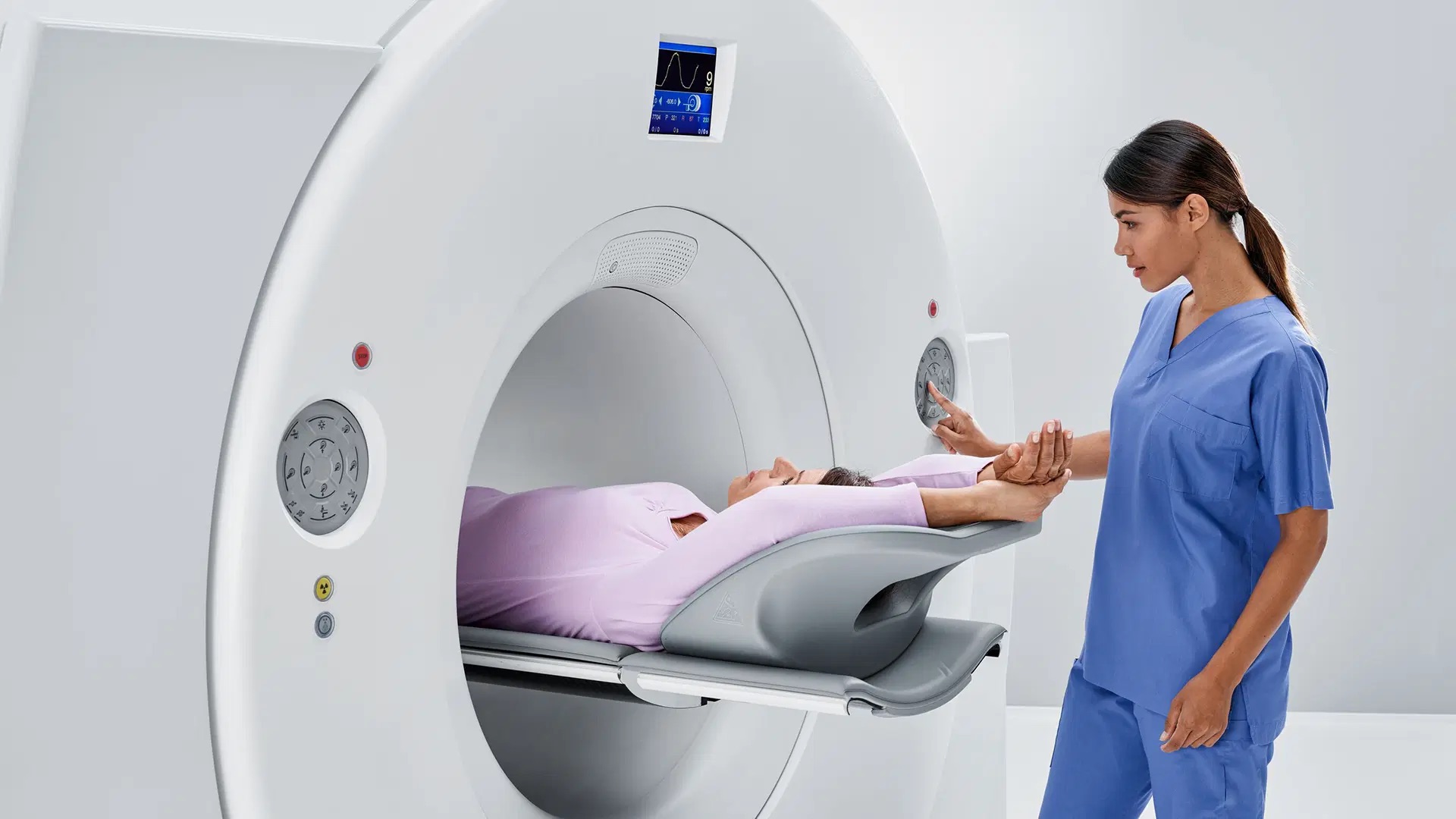
Biograph Vision.X PET/CT Scanner Cleared by FDA